The following announcement is sponsored by Galderma Laboratories, L.P.
The first-ever encapsulated benzoyl peroxide for rosacea is NOW AVAILABLE. EPSOLAY® (benzoyl peroxide) cream, 5% is now available by prescription in the U.S. for the Treatment of Rosacea Bumps and Blemishes. Here is what you need to know...
The FDA recently approved Galderma’s EPSOLAY® (benzoyl peroxide) cream, 5% for the treatment of rosacea bumps and blemishes in adults. It is now available by prescription in the U.S. If you suffer from rosacea, you may have already tried various treatments, and you may be wondering why this formulation is different. To help you make an informed choice, here is some additional information about EPSOLAY cream, 5%:
-
A groundbreaking topical treatment: EPSOLAY cream, 5% is completely different, which is why it is the first and only microencapsulated benzoyl peroxide topical treatment proven to rapidly relieve the bumps and blemishes of rosacea.1
-
Harnessing the power of BPO: The active ingredient in EPSOLAY cream is BPO. While it has been used in other products to treat skin conditions like acne, BPO may have been previously unable to treat patients with sensitive rosacea skin, but microencapsulation technology has helped changed that.1
-
State-of-the-art technology: Although BPO may have previously been thought of as too irritating for rosacea skin, the BPO in EPSOLAY cream is formulated differently. It is wrapped up in tiny capsules which allow for the gradual release of BPO while keeping a barrier between the skin and the medication.1
-
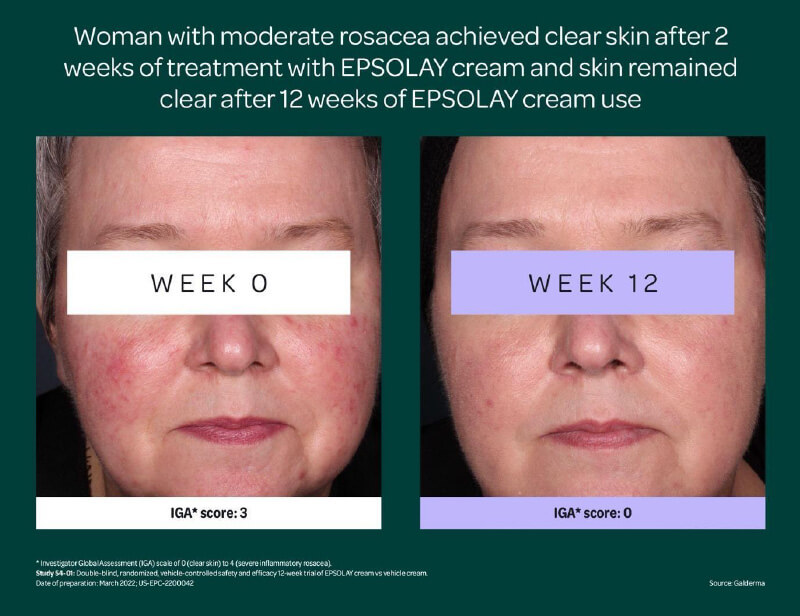
Visible results as early as 2 weeks: In clinical trials of EPSOLAY cream, people with rosacea had a 40% reduction in their rosacea bumps and blemishes in 2 weeks, and these were reduced by nearly 70% in 12 weeks. Nearly 50% of trial participants had clear or almost clear skin in 12 weeks.1,2,3
-
Unexpected tolerability: This makes it tolerable for sensitive rosacea skin.1 Even after 52 weeks of use in clinical trials, most people had mild side effects.4
-
Designed to be part of your daily routine: EPSOLAY cream is designed to be delicate on skin, so you can apply it every day as prescribed, either in the morning or evening. Using EPSOLAY cream is easy using the C-A-M-P regimen.
-
EPSOLAY cream costs as little as $0 if you are commercially covered and use a Galderma CAREConnect savings card at a participating pharmacy.
 SIGN UP to receive additional information about EPSOLAY cream and find out more at www.epsolay.com.
SIGN UP to receive additional information about EPSOLAY cream and find out more at www.epsolay.com.
If you are a prescribing dermatologist, you can find additional information at www.epsolay.com/hcp
IMPORTANT SAFETY INFORMATION
EPSOLAY® (benzoyl peroxide) Cream, 5% is indicated for the treatment of inflammatory lesions of rosacea in adults. Adverse Events: The most common adverse reactions (incidence ≥ 1%) in patients treated with EPSOLAY Cream were pain, erythema (redness), pruritus (itching) and edema (swelling), all at the application site. Warnings/Precautions: Patients using EPSOLAY Cream may experience hypersensitivity reactions, including anaphylaxis (acute allergic reaction), angioedema (rapid swelling), and urticaria (hives). If serious hypersensitivity reaction occurs, discontinue use of EPSOLAY Cream immediately and seek medical attention/initiate appropriate therapy. Skin Irritation/contact dermatitis may be experienced, including erythema (redness), scaling, dryness, and stinging/burning. Irritation and contact dermatitis may occur. Use a moisturizer and discontinue EPSOLAY Cream if symptoms do not improve. Avoid application to cuts, abrasions, eczematous, or sunburned skin. EPSOLAY Cream may increase photosensitivity, sensitivity to ultraviolet light. Minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment). Use sunscreen or protective clothing when sun exposure cannot be avoided. Discontinue use of EPSOLAY Cream at the first evidence of sunburn. You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
References:
1. Bhatia N, Werschler W, Baldwin H, et al. Efficacy and safety of micro-encapsulated benzoyl peroxide (E-BPO) cream, 5% in papulopustular rosacea: results from two phase 3, vehicle-controlled trials. Poster presented at: Maui Derm for Dermatologists; January 25-29 2020, Maui, Hawaii.
2. Galderma Laboratories, L.P., data on file. Clinical Study Report SGT-54-02, March 26, 2020.
3. Galderma Laboratories, L.P., data on file. Clinical Study Report SGT-54-01, January 8, 2020.
4. Galderma Laboratories, L.P., data on file. Clinical Study Report SGT-54-07, March 30, 2020.